Research
Design of personalized optimization of multidrug combinations
It is widely understood that future cancer therapy will be improved by the combination of existing drugs. The main problem with combination of drugs is the occurrence of side effects and the huge number of different possibilities. Therefore, current development of combination therapies is based on the physician's intuition and a trial and error approach.
Using our proprietary platform, the Therapeutically Guided Multidrug Optimization (TGMO), we screen for optimized drug-combinations on freshly isolated patient-derived organoids. We work in close collaboration with the Clinical Oncology department (HUG) and the Clinical Pathology Service (HUG), where the patient derived CRC material is diagnosed, classified, and molecularly stratified. Using different mathematical modeling tools, including second order linear regression analysis and penalized regression, the most interesting and synergistic interactions between drugs are identified, leading to the optimization of synergistic, low-dose, selective, patient-specific drug mixtures. With our collaborators at the Ecole Polytechnique Fédérale de Lausanne (EPFL), we are able to perform whole exome sequencing and RNA data analysis to define the stage of the tumor and the main driver mutations in each patient material, which gives us insight on the mechanism of action of the optimized patient-specific drug mixture.
This project represents a proof-of-concept of a platform for designing precision treatment strategies for a facilitated translation of treatment to be tailored specifically to individual patients. The strength of the proposed approach is based on a “from bench to bedside and back” strategy carried out by specialists from fundamental researchers and clinicians. The proposed approach will, for the first time, lead to the rapid optimization of synergistic multi-drug combination therapy tailored specifically to individual patients. By selecting drugs for which clinical data are already available, a rapid progression to phase I/II clinical trials for the optimized drug mixture is envisioned.
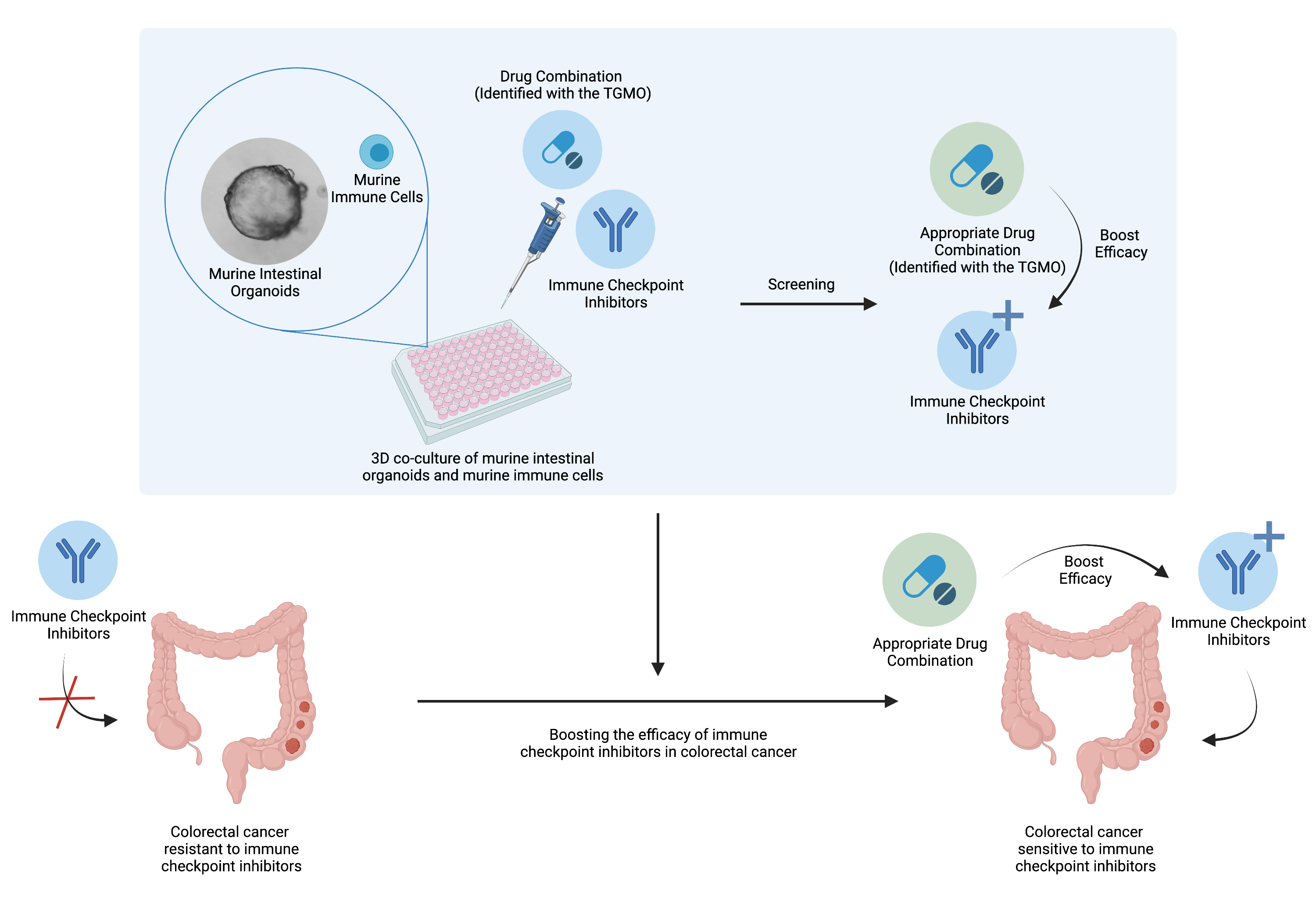
Drug combinations to boost the activity of immune checkpoint inhibitors
Complex in vitro platforms to mimic tumor microenvironment
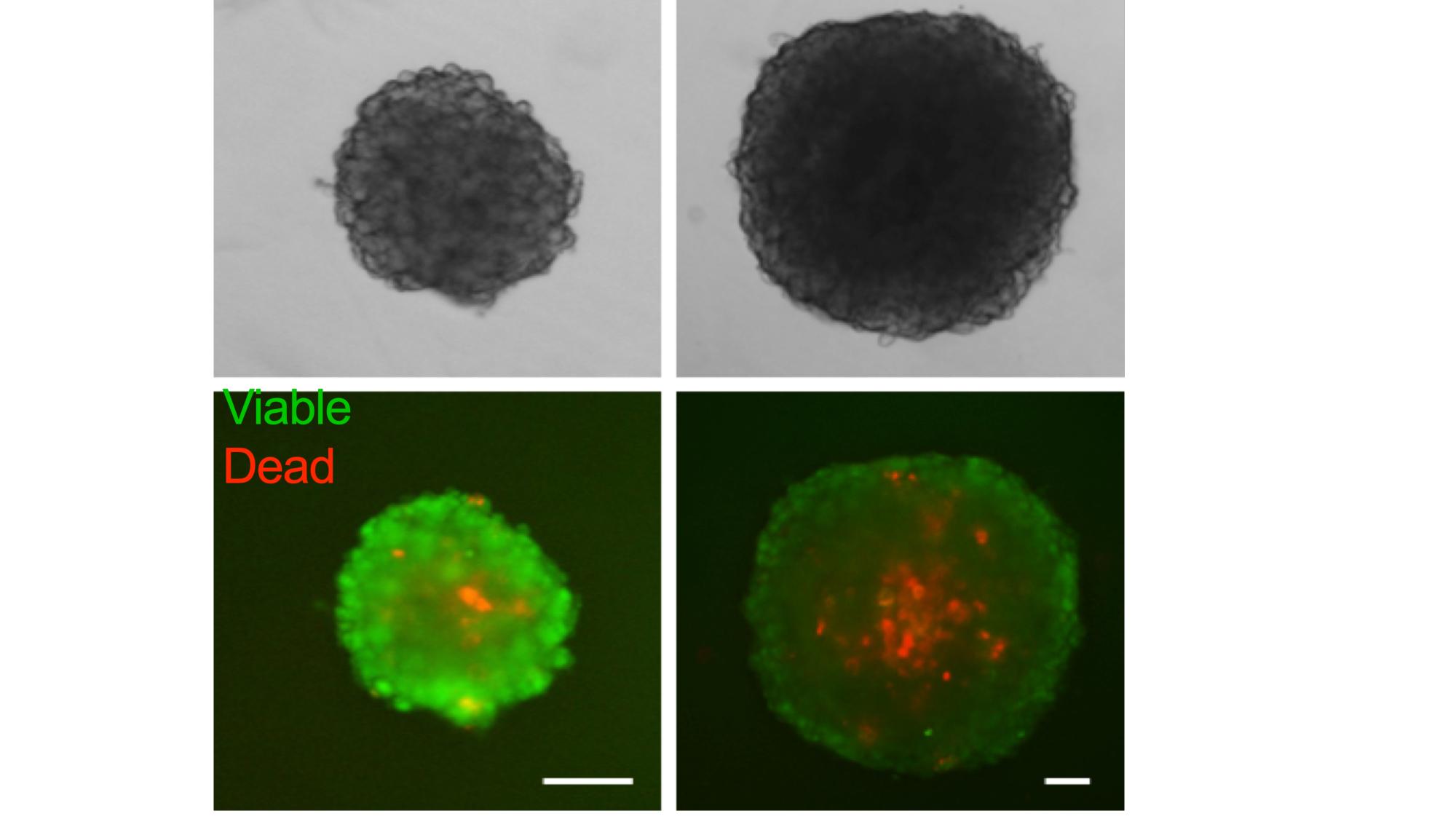
We established experimental platforms to mimic human tumors in laboratory settings. It is known that a tumor contains not only cancer cells, but entire microenvironment that is composed of the cellular and the non-cellular compartment. Therefore, we developed 3-dimensional heterotypic co-cultures that contain cancer cells, endothelial cells, fibroblasts, and selected immune cell subsets (Scientific Reports 2019; Cancers 2019, Cancers 2020). Together, these cells produce an extracellular matrix promoting the 3-dimensional structure formation. We use these co-cultures to (i) validate the anti-cancer efficacy of optimized multidrug combinations, (ii) characterize the selective targeting of cancer cells, (iii) monitor the behavior of the cells in response to the applied treatment, (iv) and more.
Drug combinations and metabolomic analysis
The value of metabolome analysis (metabolomics) has been redefined from a simple tool for identifying biomarkers to a technology for uncovering the active drivers of biological processes. Metabolites have a clear function in the life of the biological system and are also contextual, reflecting the surrounding environment. Their snapchot thus enables us to better understand metabolic pathways and subsequently to deduce the possible mechanisms of action of the drug combinations developed in our laboratory.
Acquired drug resistance against targeted- or chemo- therapy
One of the major problems in cancer treatment is the development of drug-acquired resistance. Via chronic treatment with drugs, we have generated treatment-resistant cell lines to study activity of optimized drug combinations (Cancers 2020; Molecules 2020). In the case of renal cell carcinoma (RCC), the resistance to sunitinib, a tyrosine kinase inhibitor used to treat patients with advanced RCC, was developed in several cell lines by chronic treatment. The resistance to FOLFOXIRI, the 1st line treatment for colorectal carcinoma (CRC) patients, was developed in several CRC cell lines.
After the development of these treatment-resistant cell lines, we focus on studying their vulnerability to other treatments and drug combinations, along with their characterization by RNA sequencing to highlight the up- and down-regulated genes (Cancers 2022).
These treatment-resistant cell lines can further be used to perform TGMO-based drug combination optimizations and screen for drug combinations that can overcome the effect of the chronic resistant induction.
Drug combination delivery through a nanocarrier
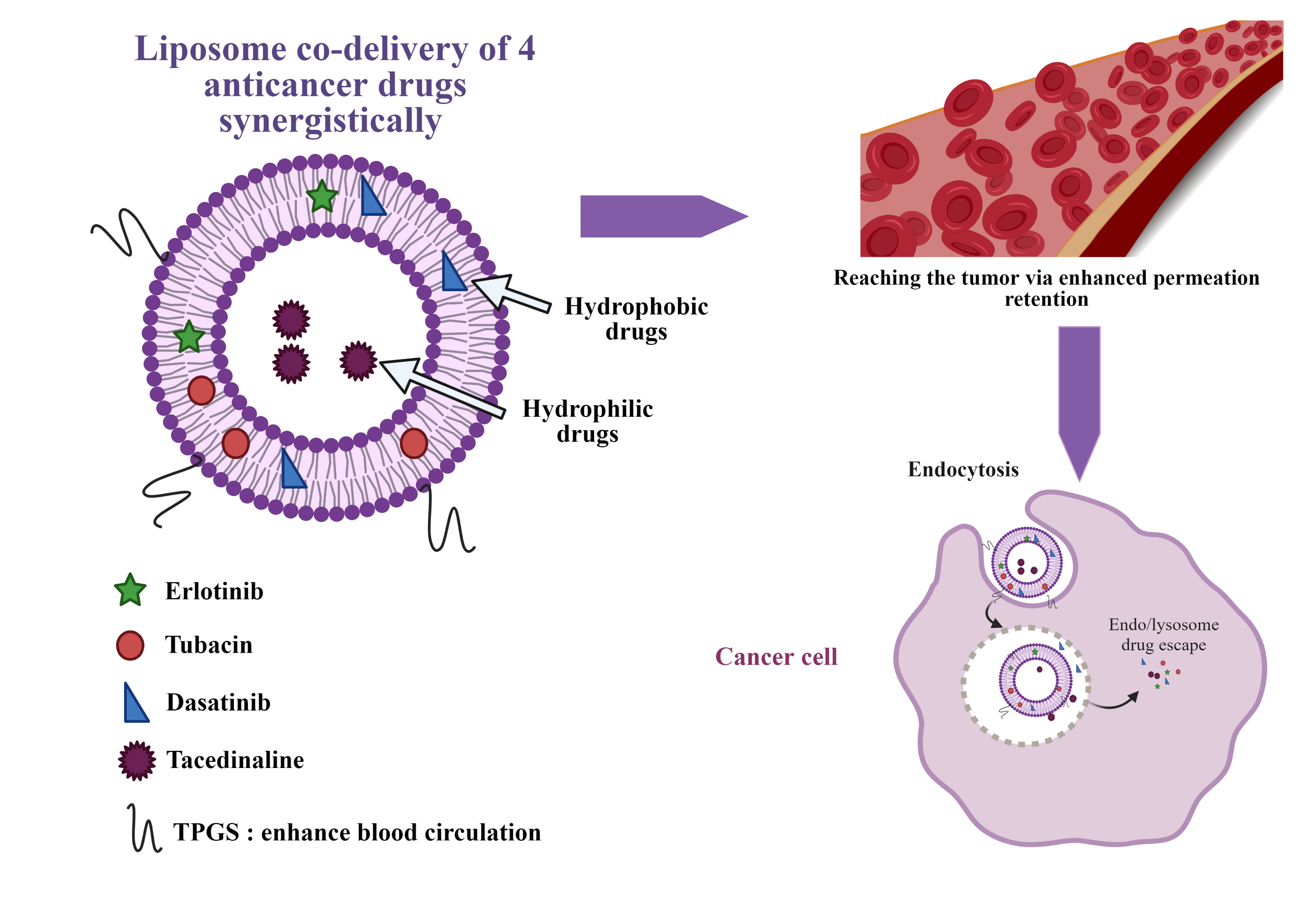
One common strategy of treatment of complex diseases, such as AIDS, diabetes, hypertension or bacterial infections is the use of drugs combination. Our lab identified a four-drug combination active in cancer treatment in a synergy-dependent-ratio. However, upon injection, due to different pharmacokinetics of the drugs in combination, the ratio might no longer be respected.
To address this challenge, the optimized four-drug combination will be encapsulated in a nanocarrier, granting a protection from blood proteins with a prolonged blood circulation, reducing side effects while maintaining the drug ratio until it reaches the cancer cell. To this purpose, liposomes are a nanocarrier of choice: they are biocompatible and allow simultaneous encapsulation of hydrophilic and hydrophobic drugs. This project is performed in collaboration with the group of Prof. Gerrit Borchard (ISPSO, UNIGE) and the recent review article in this domain was published by us in the Journal of Controlled Release: https://www.sciencedirect.com/science/article/pii/S0168365923002572?via%3Dihub