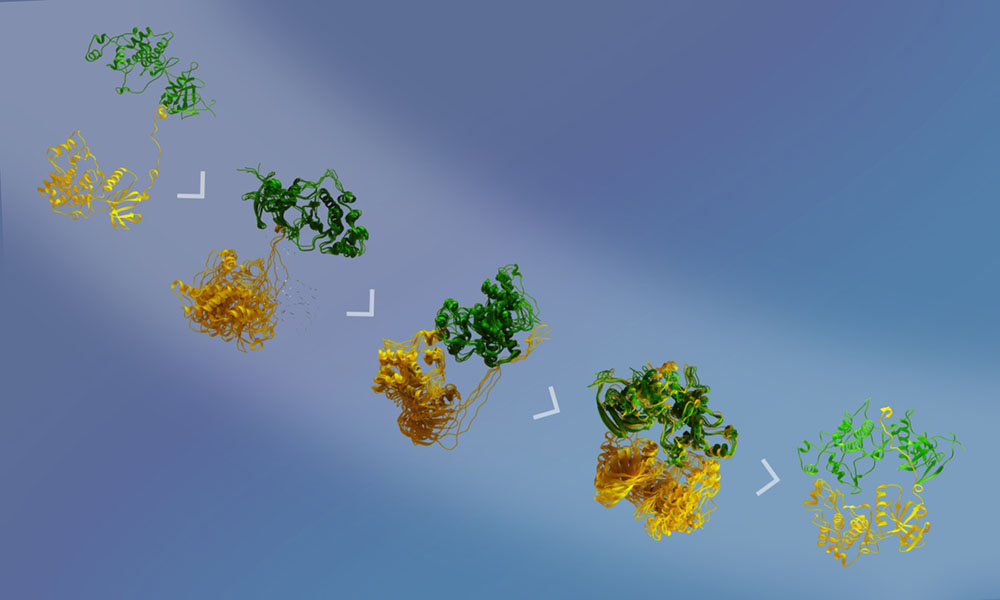
Mitogen-activated protein kinases (MAPKs) are a key player in cellular responses to various stimuli in eukaryotes. Signaling cascades occur through a series of upstream kinases, eventually resulting in double phosphorylation of MAPK that occurs in a complex that is transient and dynamic and thus difficult to visualize by traditional structural approaches. Juyoux et al. combined cryo–electron microscopy, biophysical techniques, and molecular dynamics simulations to construct a model of the active complex between the MAPK p38α and its upstream kinase, MKK6. Based on this model, the authors discuss specific interactions, selectivity, and the overall mechanism of p38α activation. These findings will be important for researchers seeking to target MAPKs for drug development. —Michael A. Funk (Science editor).
P. Juyoux, I. Galdadas, D. Gobbo, J. Von Velsen, M. Pelosse, M. Tully, O. Vadas, F. L. Gervasio, E. Pellegrini, M. W. Bowler. Architecture of the MKK6-p38α complex defines the basis of MAPK specificity and activation. Science 2023 Vol 381, Issue 6663.
doi.org: DOI: 10.1126/science.add7859.
Abstract:
The mitogen-activated protein kinase (MAPK) p38α is a central component of signaling in inflammation and the immune response and is, therefore, an important drug target. Little is known about the molecular mechanism of its activation by double phosphorylation from MAPK kinases (MAP2Ks), because of the challenge of trapping a transient and dynamic heterokinase complex. We applied a multidisciplinary approach to generate a structural model of p38α in complex with its MAP2K, MKK6, and to understand the activation mechanism. Integrating cryo–electron microscopy with molecular dynamics simulations, hydrogen-deuterium exchange mass spectrometry, and experiments in cells, we demonstrate a dynamic, multistep phosphorylation mechanism, identify catalytically relevant interactions, and show that MAP2K-disordered amino termini determine pathway specificity. Our work captures a fundamental step of cell signaling: a kinase phosphorylating its downstream target kinase.
Press Release:
· Unige.ch (en): Understanding the ''dance'' of signalling proteins to stop inflammation
· Miragenews.com (en): Decoding Protein Dance to Halt Inflammation
· Eurekalert.org (en): Switching off the cytokine storm
· MyScience.ch (en): Understanding the ’’dance’’ of signalling proteins to stop inflammation
